Why Should a Magnesium Ribbon be Cleaned Before Burning in Air: What You Need to Know
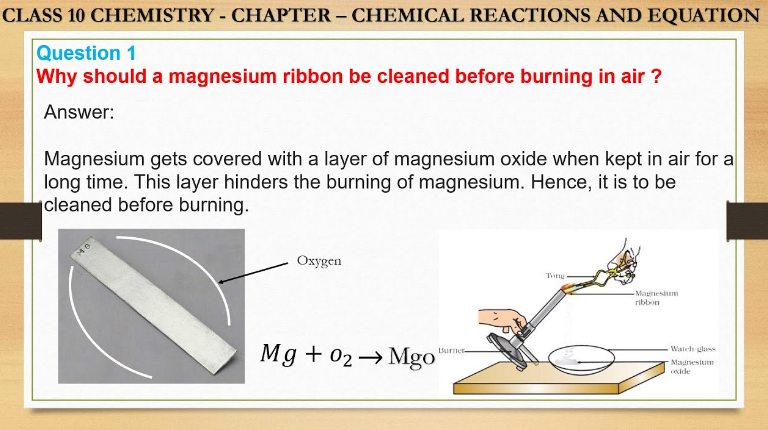
Before burning in the air, a magnesium ribbon should be cleaned to remove any oxide layer, ensuring a more efficient reaction. Cleaning the magnesium ribbon enhances its reactivity by exposing fresh metal surfaces for better combustion when ignited.
This process helps prevent impurities from interfering with the reaction and ensures a more complete combustion of the magnesium ribbon, resulting in a brighter and more vigorous flame. By cleaning the magnesium ribbon, you optimize its performance and ensure a successful and impactful demonstration of its characteristic bright white light when burned in the air.
This simple precaution can significantly enhance the overall experience and educational value of observing the combustion of magnesium.
Contents
Chemical Properties Of Magnesium
Magnesium’s chemical properties make it an interesting element to study. It is a highly reactive metal that easily forms compounds with other elements. Magnesium also possesses a high combustibility, making it ideal for various chemical applications and experiments.
High Reactivity
Magnesium is known for its high reactivity, readily reacting with numerous environmental substances. When exposed to air, magnesium quickly forms a thin layer of oxide on its surface, which protects it from further oxidation. This reactivity makes it crucial to clean magnesium ribbon before burning in the air, ensuring that the pure metal reacts as expected.
Combustibility
Magnesium’s combustibility is a notable property, as it ignites easily and burns with a bright, intense flame. This high flammability is due to the metal’s ability to react exothermically with oxygen, releasing much energy through heat and light. As a result, precautions must be taken when handling and burning magnesium to prevent accidents or uncontrolled reactions.
Explosive Potential Of Magnesium Ribbon
Magnesium ribbon possesses explosive potential due to its reaction with oxygen in the air. Understanding this reaction is crucial for handling magnesium safely.
Reaction With Oxygen
When exposed to air, magnesium ribbon rapidly reacts with oxygen in the atmosphere, leading to a highly exothermic reaction.
Formation Of Magnesium Oxide
The rapid combustion of magnesium ribbon results in the formation of magnesium oxide, a white powdery substance that covers the ribbon’s surface.
Importance Of Cleaning Magnesium Ribbon
Cleaning magnesium ribbon before burning in the air is crucial to ensure efficient combustion and avoid unwanted side reactions. By removing any impurities or oxide layers, the clean surface of the ribbon allows for a more controlled and complete reaction with oxygen, resulting in a more effective burning process.
Removal Of Oxidized Layer
Cleaning magnesium ribbon helps in removing the oxidized layer, which hinders combustion.
Enhancing Reactivity
Cleaning magnesium ribbon boosts reactivity by exposing fresh metal surfaces to oxygen.
Cleaning magnesium ribbon is crucial before burning in the air to ensure optimal combustion.
Removing the oxidized layer helps in enhancing reactivity for better combustion results.
Methods To Clean Magnesium Ribbon
Magnesium ribbon is commonly used in chemistry experiments and demonstrations because it can burn brightly in the air. However, cleaning the magnesium ribbon is essential to ensure a successful reaction before burning. The cleaning removes any impurities or oxide layers on the surface, allowing for a clean and efficient burn.
Using Sandpaper
One effective method to clean magnesium ribbon is by using sandpaper. Take the ribbon and gently rub it with fine-grit sandpaper to remove any impurities or oxide layers. This process helps to expose the clean metal surface, ensuring a more efficient and controlled burn when ignited.
Chemical Cleaning Agents
Another method of cleaning magnesium ribbon is to use chemical cleaning agents. One popular choice is to immerse the ribbon in a diluted acid solution, such as hydrochloric acid. This helps dissolve any oxide layers or impurities on the ribbon’s surface, leaving behind a clean metal surface ready for burning.
Safety Precautions
Proper Handling Of Magnesium Ribbon
Proper handling of magnesium ribbon is crucial to ensure your safety during the burning process. Before you begin, make sure you have a clear understanding of how to handle the ribbon safely. This includes wearing appropriate protective gear, such as gloves and goggles, to protect your skin and eyes from potential hazards. Taking these precautionary measures can minimize the risk of accidents or injuries.
Avoiding Contact With Moisture
Avoiding contact with moisture is essential when working with magnesium ribbon to prevent any unexpected reactions. Moisture can react with magnesium and produce flammable hydrogen gas, which could lead to dangerous situations. Keeping the ribbon dry and storing it in an airtight container is essential. This way, you can ensure that moisture does not come into contact with the ribbon and risk any potentially hazardous incidents.
To avoid mishaps, always store and handle the magnesium ribbon properly. Proper storage ensures that the ribbon remains moisture-free and stays in optimal condition for future use.
Summary:
- Proper handling of magnesium ribbon is crucial to ensure safety.
- Wear protective gear such as gloves and goggles.
- Avoid contact with moisture to prevent unexpected reactions.
- Keep the ribbon dry and store it in an airtight container.
- Proper storage ensures the ribbon remains in optimal condition.
Following these safety precautions, magnesium ribbon can be safely used for various purposes while reducing the risk of accidents and maintaining a controlled environment. Always prioritize safety when handling potentially hazardous materials like magnesium ribbon.
Conclusion
Cleaning a magnesium ribbon before burning it in the air is crucial for safety and efficiency. Removing the oxide layer ensures a more vigorous reaction, producing a brighter flame and more accurate experiment results. By following this important step, you can ensure the success of your chemistry experiments and avoid potential hazards.