How Many Valence Electrons Does Chlorine Have? Find Out Now!
Chlorine has seven valence electrons, which determine its reactivity and chemical properties. These electrons are located in the outermost shell of the chlorine atom.
Understanding the number of valence electrons in an element like chlorine is essential in predicting its behavior when it forms chemical bonds with other atoms. By knowing the valence electrons, scientists can predict how chlorine will interact with other elements and form compounds.
Overall, the seven valence electrons of chlorine play a crucial role in its chemical reactivity and ability to form various compounds in nature.
Credit: m.youtube.com
Contents
What Are Valence Electrons?
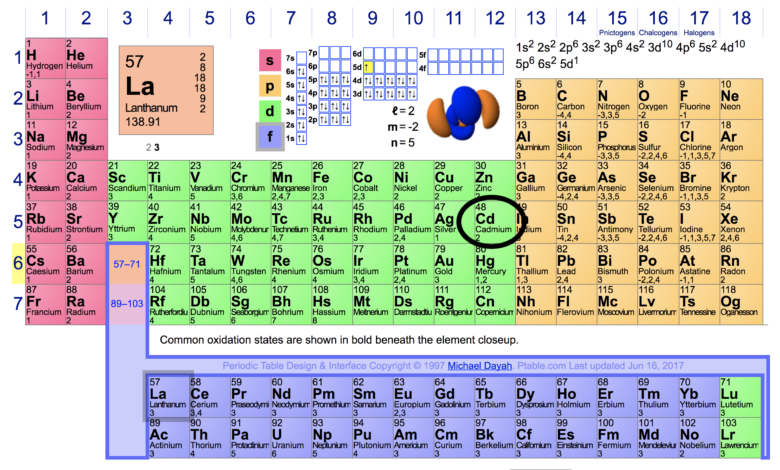
Chlorine has 7 valence electrons. Valence electrons are the electrons in the outermost energy level of an atom and are responsible for the atom’s chemical properties and ability to form bonds with other elements.
Definition Of Valence Electrons
A valence electron is the electron present in the outermost shell of an atom. These are the electrons involved in chemical bonding because they are the furthest from the nucleus and thus have the strongest interaction with other atoms.
Importance Of Valence Electrons
The number of valence electrons determines the chemical properties of an element. For instance, chlorine has seven valence electrons, making it highly reactive. Understanding valence electrons helps predict how elements will behave when they form compounds.
Credit: chem.libretexts.org
Understanding Chlorine
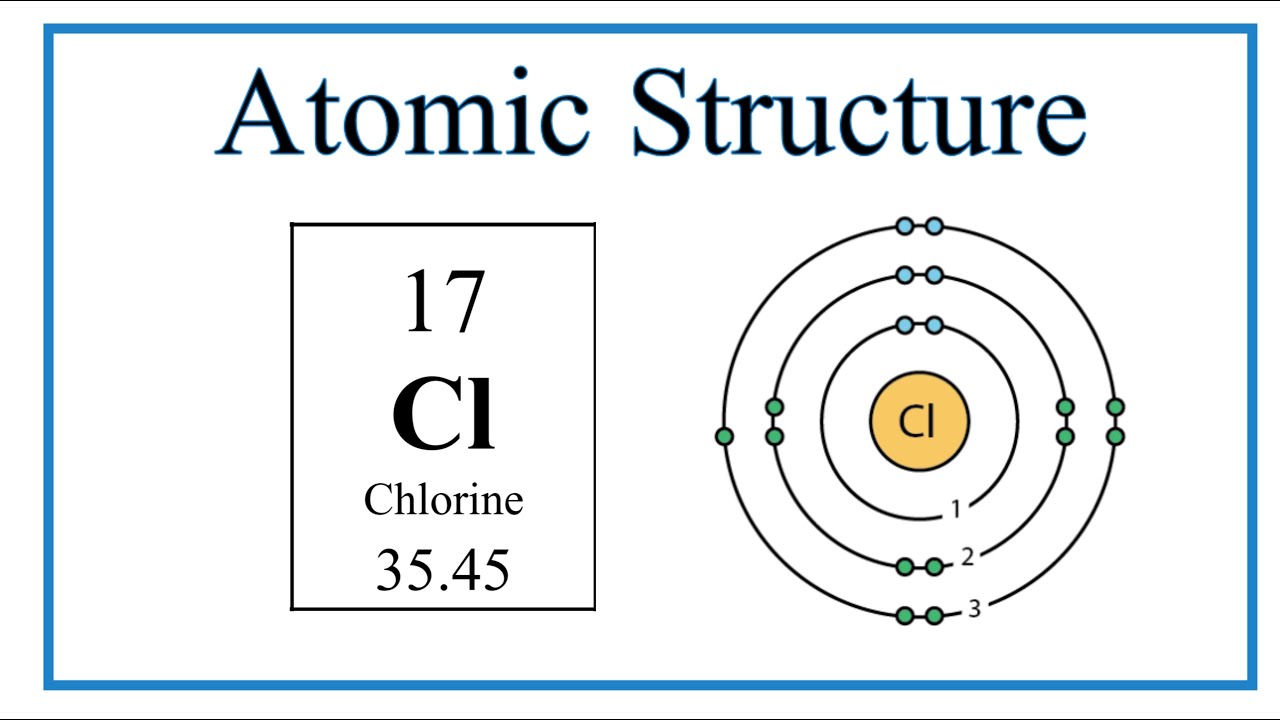
Chlorine has seven valence electrons, making it a halogen group element. Its electron configuration is 2,8,7, meaning it has 7 electrons in its outer shell. This makes it highly reactive, and it tends to gain one electron to achieve a stable octet configuration.
Overview Of Chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17.
It belongs to the halogen group in the periodic table and is a highly reactive nonmetal.
Atomic Structure Of Chlorine
Chlorine typically has 17 protons and electrons in its atomic structure.
It contains 7 valence electrons in its outermost shell, making it highly reactive.
Valence electrons are responsible for forming chemical bonds with other elements.
Determining The Number Of Valence Electrons In Chlorine
Chlorine has seven valence electrons, as indicated by its position in Group 17 of the periodic table. Valence electrons play a crucial role in chemical bonding and reactions. By understanding the number of valence electrons in chlorine, scientists can predict its behavior in various chemical reactions with accuracy.
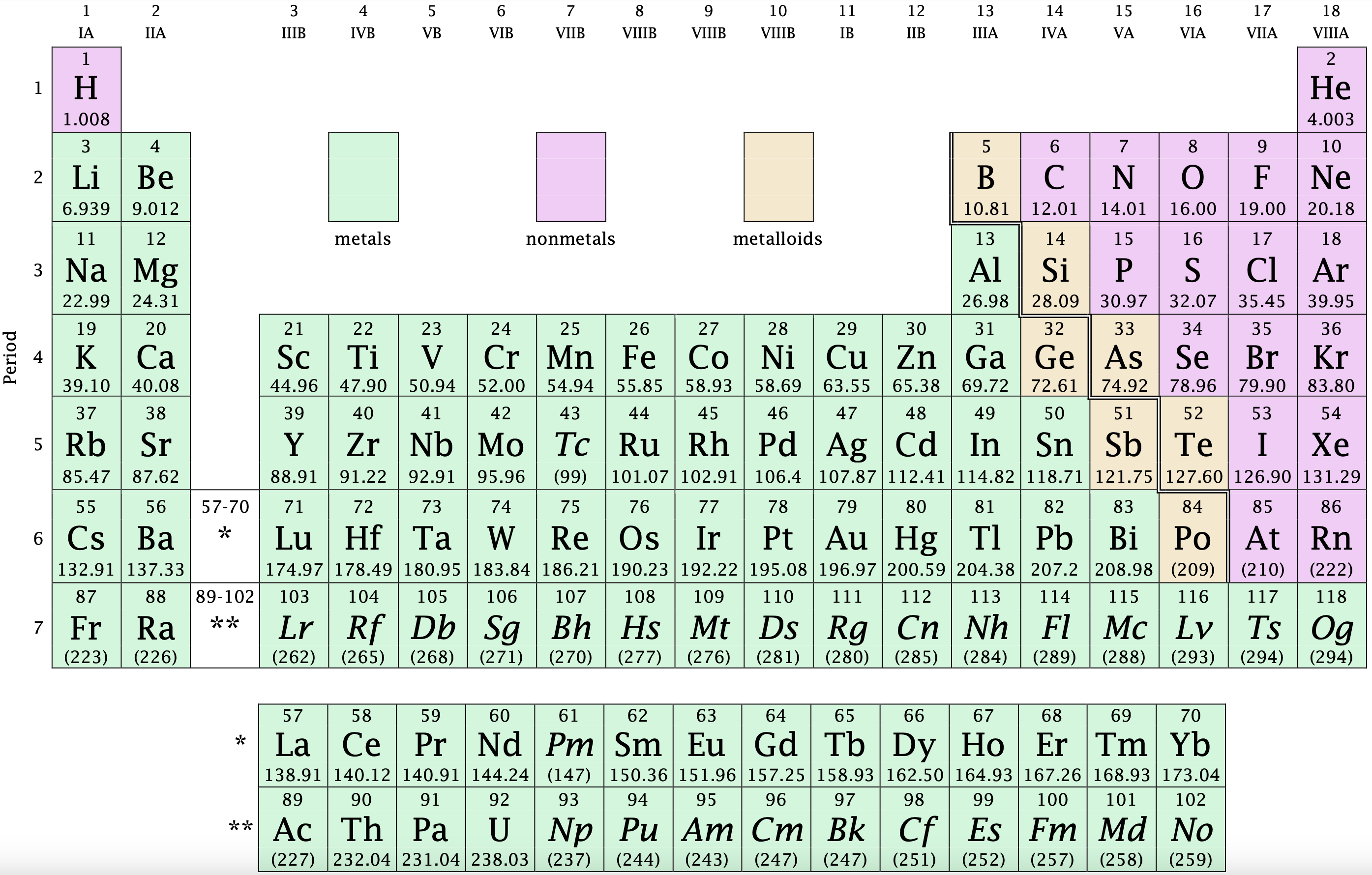
Using The Periodic Table
On the periodic table, the number of valence electrons in an element can be determined by looking at the group number where the element is located. For non-transition metals, the group number directly corresponds to the number of valence electrons.
Group Number Method
The group number method simplifies the process of finding the valence electrons for elements like chlorine. By identifying chlorine’s group number, which is 7, you can conclude that chlorine has seven valence electrons.
Lewis Dot Structure Of Chlorine
Chlorine has 7 valence electrons, which are crucial for forming chemical bonds with other elements. By referencing the periodic table’s group number, you can quickly determine the number of valence electrons in a chlorine atom. The Lewis Dot Structure for chlorine showcases these valence electrons.
The Lewis Dot Structure of Chlorine The Lewis dot structure of an atom represents the valence electrons present in the outermost shell. For Chlorine, the Lewis dot structure helps in understanding the arrangement of its valence electrons. This structure aids in visualizing how the electrons are shared and bonded with other atoms when forming compounds.
Explanation of Lewis Dot Structure The Lewis dot structure is a visualization of the arrangement of an atom’s valence electrons. It uses dots to represent the individual valence electrons around the atomic symbol. This method enables us to see the outermost electrons and understand how they interact with other atoms during chemical reactions.
Finding Valence Electrons using Lewis Dot Structure To find the valence electrons of Chlorine using its Lewis dot structure, reference the periodic table. Chlorine belongs to Group 17, also known as the halogens. This means it has 7 valence electrons.
By representing Chlorine’s symbol “Cl” and placing 7 dots around it in a circular pattern, we can easily visualize the arrangement of its valence electrons. This representation facilitates understanding the bonding behavior of Chlorine in various chemical reactions.
By utilizing the Lewis dot structure, knowing how many valence electrons Chlorine has becomes simpler. This visual aid helps in comprehending the reactivity and bonding characteristics of Chlorine, making it a valuable tool in chemistry education and research.
Valence Electrons In Chlorine Ion
Chlorine ion contains 8 valence electrons. Although the natural state of chlorine has 7 valence electrons, it gains an extra electron when it forms an ion, resulting in a full octet and 8 valence electrons. This makes the chlorine ion more stable and less reactive.
Chlorine is a highly reactive nonmetal element with atomic number 17. It belongs to Group 17, also known as the halogens, in the periodic table. Chlorine, in its neutral state, has 7 valence electrons. However, the valence electrons in a chlorine ion may vary depending on whether it gains or loses electrons to achieve a stable electronic configuration.
Total Number Of Valence Electrons In Chlorine Ion
When chlorine gains one electron, it forms a negatively charged chloride ion (Cl-). In this process, the chlorine atom achieves a stable octet configuration, similar to the noble gas argon (Ar). As a result, the chlorine ion now possesses 8 valence electrons, making it more stable and less reactive than its neutral counterpart.
To recap, a neutral chlorine atom has 7 valence electrons, whereas a chloride ion has 8 valence electrons due to the addition of an extra electron. Understanding the valence electrons in chlorine and chloride ions is crucial in predicting their chemical behavior and reactivity with other elements. Now that we have explored the valence electrons in a chlorine ion, let’s dive deeper into their significance and how they influence chemical reactions and bonding.

Credit: byjus.com
Conclusion
Understanding the number of valence electrons in chlorine is crucial for comprehending its chemical behavior. With seven valence electrons, chlorine readily forms bonds with other elements to achieve a stable electron configuration. This knowledge is fundamental in various fields, including chemistry, physics, and material science.
Delving into the intricacies of valence electrons enhances our understanding of the fundamental building blocks of matter.